Synthetic diamonds and tools based on them are science-intensive products. According to the mo-dern market defi nition, they can be attributed to the medium or high-tech industry that include aircraft, automobile and instrument engineering, pharmaceuticals, and chemical industry. One of the world centers for diamond business in Ukraine is the Bakul Institute of Superhard Materials of the National Academy of Sciences of Ukraine. The R&D activity of the Institute is aimed at laying scientific foundations for the creation of cutting-edge technologies for processing metals and non-metals with tools made of hard alloys and superhard materials (SHM) and at developing methods and technologies for the use of new tool materials in the basic industries. The in-dustrialized countries have been more and more focusing on SHM, as the most eff ective for equip-ping tools, and this has been evidenced by the fact that now the leading industrialized economies (USA, Japan, Germany, England, Italy, France, and China) use about 80% of mined natural and manufactured synthetic diamonds. At the same time, one of the main areas of application of SHM is mechanical processing, in which about 70% of the total SHM is used.
In the previous research published in Science & Innovation journal of the National Academy of Sciences of Ukraine [1], we have considered im-proving the operational characteristics of high-strength synthetic diamonds AS65–AS250 for high-precision diamond ruling tools. In this re-search, we consider improving the operational characteristics of a diamond grinding tool that uses diamonds of lesser strength. Thus, AS6–АС20 diamond synthetic powders have been widely employed in diamond grinding tools in the industry for machining products made of hard alloy, ceramics, glass, and other fragile materials. The fur-ther development of modern diamond machining technologies is associated with the use of pow-ders with new unique properties, special grain morphology, and increased chemical and thermal resistance in diamond tools. Thus, one of the causes for the increased consumption of diamonds in the operation of the tool, as well as in the manufacture of some types of tools on a metal binder is oxidation of diamonds under high temperature. The development of effective methods for in-creasing the heat resistance of grinding powders made of superhard materials, including abrasive grinding powders made of synthetic diamond powders, contributes to improving the quality of grinding tools. To increase the heat resistance of diamonds, they are covered with a metal (metallization) or ceramic layer, and alloying additives of certain elements are introduced into the reaction mix used in the synthesis of diamonds. Other coating methods have also been developed to in-crease the heat resistance of diamonds, such as: glass coating, vacuum ion-plasma sputtering, epitaxial synthesis, magnetron sputtering, and liquid phase coating method. That is, modification of the surface, or coating, of diamond grains is one of the important factors influencing changes in their properties, increasing retention in the binding working layer of the grinding tool, and changing the properties of the diamond surface. It should be noted that this direction has been intensively developing. Let us further focus on modern developments in the production of various functional coatings on diamonds and the features of their influence on the modification of the surface of diamonds.
The technology of thermo-explosive synthesis can be applied to cover diamond [2]. With the use of mixed Cr/Al/B/diamond powder as a raw ma-terial, a multi-component composite coating based on CrB-AlN has been formed on the sur-face of the diamond by the thermo-explosive syn-thesis method (Fig. 1). The eff ect of the protec-tive atmosphere (N or Ar), the content of Al in the phase composition, and the microstructure of the binder and coating have been studied. The re-sults have shown that under the protection of Ar, the raw material does not undergo a thermal ex-plosion reaction. The loose and porous bulk structure can be obtained by the thermal explo-sion reaction under the protection of N. The coat-ing on the diamond surface is mainly composed of CrB and AlN and contains other by-products such as Cr5Al8 and Cr2AlB2 [2].
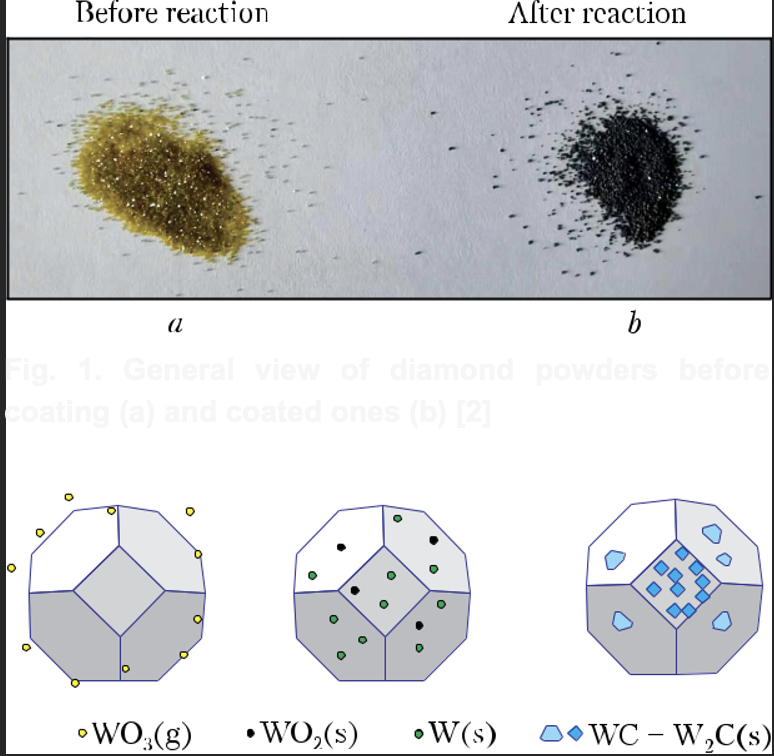
Metallic W-containing coatings are applied to the surface of diamond microcrystals by hot pres-sing, with the use of WO3 as a metal source [3]. The coatings formed on the diamond surface re-act with WO3 powder during hot pressing at ele-vated temperature. After processing the mix at 850 C for 15 min, WO2 and W18O49 are fi xed on the diamond surface. After treatment at 950 С (15 min), tungsten is the dominant phase of the coating. Treatment at 1150 С (15 min) leads to the formation of WC and W2C phases. There is no metallic tungsten in the coating obtained at this temperature (Fig. 2).In [4], to determine the features of the reaction of diamond with various types of metals and met-al oxides, the activation energy of the reaction between diamond and metals, as well as between diamond and metal oxides, has been calculated from the fi rst principles. For the transition metals of the fourth period of the Periodic Table of the Elements, when they react with diamond to form the corresponding metal carbides, the order of in-creasing activation energy for the metals is as fol-lows: Mn, Fe, V, Ti, Cr, Co, Ni, Zn, and Cu. And when diamond reacts with MnO, FeO, CoO, NiO
and CuO to form metals and CO, the calculated activation energy is, in the descending order, as follows: MnO, FeO, CoO, NiO, and CuO. Thus, it has been established that NiO and CuO are reduced by diamond to Ni and Cu, which indicates a redox reaction between diamond and metal oxides [4].
In [5], diamond particles are coated with thin layers of aluminum oxide (Al2O3) by the atomic layer deposition method. As a result, the temperature at which the diamond starts decomposing to CO2 shifts towards higher temperature (by ≈ 50 K) due to the protective effect of Al2O3. The authors of [5] have stated that although the improvement is small enough to be used for high-temperature applications, these results have indicated that this type of coating can be used to protect diamond from oxidation. Efficient control of interphase electronic states of Al2O3/diamond interfaces has been considered in [6], where calculations have been used to find out the influence of Al2O3 and diamond planes on the electronic properties of Al2O3/diamond interfaces. Thus, for the most stable C—Al interface
in Al, under conditions of increased Al content, there is achieved strong p-type doping with obvi-ous localization characteristics due to a charge transfer generated at the C—Al interface.
However, the pure diamond coating has a low impact toughness because of its superhardness, which may cause it to peel or break. In [7], a new method of chemical vapor deposition when a diamond coating with CuO particles is applied to a WC/Co substrate has been proposed. A clean diamond coating has been produced for comparison. The indentation tests have shown that the diamond coating with CuO particles has higher adhesion strength and cracking resistance as compared with the pure diamond coating.
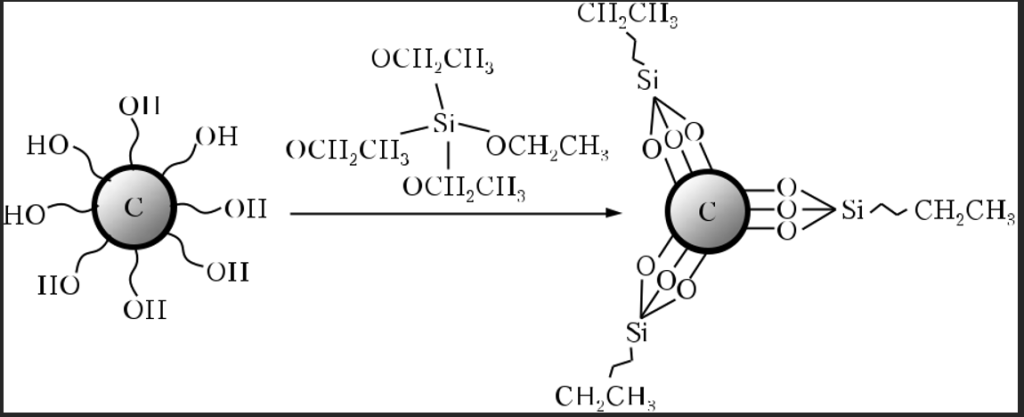
In [8], in order to improve the dispersion and adhesion strength in the polishing tool, the sur-face of diamond abrasives has been modifi ed by applying a layer of SiO2 by the isothermal hydrol-ysis method. The results have shown that a thin SiO2 fi lm is uniformly grafted to the diamond surface (Fig. 3). Polishing films have been produced with the use of unmodified and modified diamonds as an abrasive based on the Sol-Gel technology for polishing SiC substrates. The tests have shown that the diamond abrasives modifi ed with SiO2 have a higher material remov-al rate and better polishing quality than the un-modifi ed diamond abrasives. This is caused by an increase in the dispersive ability of abrasive grains and an improvement in the adhesion be-tween the fi lm matrix and abrasives [8].
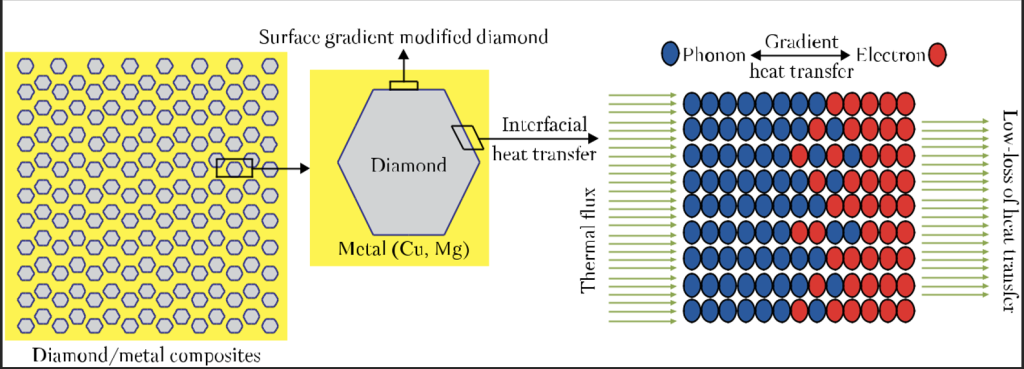
Research [9] presents an innovative method of molten salts for the reactive preparation of surface-modified diamond particles. Surface modified composites based on metals (copper or magnesium), reinforced with diamond particles have been obtained by the electrospark plasma sintering method. Diamond particles with a modified surface gradient have been obtained by the method of melting salts (Fig. 4). The results have shown that the thickness of the modified layer on the diamond surface gradually grows as the exposure time increases. The composite materials on a met-al base with a modified surface, reinforced with diamond particles, have a high thermal conductivity. With a volume fraction of diamond of 35%, the thermal conductivity of diamond-copper composites reaches 602 W/(m · K), while that of diamond magnesium ones is equal to 286 W/(m · K).
In [10], the behavior of pure diamond powders and powders containing 0.2% wt. boron during oxidation has been studied. The boron-doped diamond has shown a significantly higher resistance to oxidation than the pure diamond. The maximum oxidation rate shifts from 773 C for the pure diamond to 1118 C for the boron-doped diamond. The SEM analysis of the surface of par-tially oxidized diamonds has proven that even such a low boron content (0.2%) is sufficient for the formation of a protective B2O3 layer in the ar-eas of active oxidation. According to the authors of [10], it is this layer that is the cause of increased resistance to oxidation.
To some extent, this has been confirmed in [11], where protective coatings made of titanium boron carbide on diamond particles have been studied. The results have proven that the boron con- tent is important for the adhesion of Ti in the Ti—B—C coating. Such a coating with a boron content of 60 at. % protects the diamond from oxidation for more than 1 h, in the course of to 1000 C, in the air. In the case of the Ti—B—C coating with a boron content of 11 at. %, the diamond mass after heating to 1000 C decreases. During annealing of coated diamond in the air, the a priori formed B2O3 and TiO2 protect the diamond from oxidation, acting as oxygen-impermeable layers. In addition, the formation of liquid B2O3 allows avoiding the delamination of TiO2, as a result of volume expansion during oxidation. Meanwhile, the presence of TiO2 provides a long-term protection by reducing the evaporation of B2O3 [11]. It can be seen from the above that various oxides are used to modify the surface of diamonds: WO2, NiO and CuO, Al2O3, SiO2 and B2O3. At the same time, in [12], the authors have stated that for the cutting processes, oxygen is an important factor in terms of modifying the tribotechnical characteristics of contact surfaces. The oxide layers arising under the action of oxygen or its compounds on contact surfaces significantly reduce friction. The higher the chemical activity of the metal towards oxygen, the greater the effect of friction reduction. Meanwhile, let us return to the influence of oxides on the oxidation of diamonds. In [13], it has been determined which ox- ides may act so and has been shown that the metal oxides rather than the very metals have a decisive influence on the heat resistance of diamonds. In addition, since diamonds are pressed into the bond of the working layer, it is desirable that such oxides have neither a high coefficient of thermal expansion (CTE) nor a large specific heat capacity. At the same time, it is known [13] that for the metals, the higher the melting point, the lower the CTE. In [13], for the oxides, an inverse relationship has been observed: as the melting temperature (Tpl) increases, so does the CTE ( ) (Fig. 5). Figure 5 shows that the oxides of metals of the VIII group (iron, cobalt and nickel), as well as germanium occupy a separate area parallel to the main one. A similar grouping has been also observed in the analysis of the relationship between the CTE and the standard molar heat capacity of oxides [13]. The analysis of the relationship between the density and the heat capacity has shown that the standard heat capacity goes up slightly as the density of oxides increases. A significant increase has been observed for the oxides with the composition of Me2O3. In general, the above-described grouping by the oxide metal valence has been reported. Therefore, oxides have many characteristic
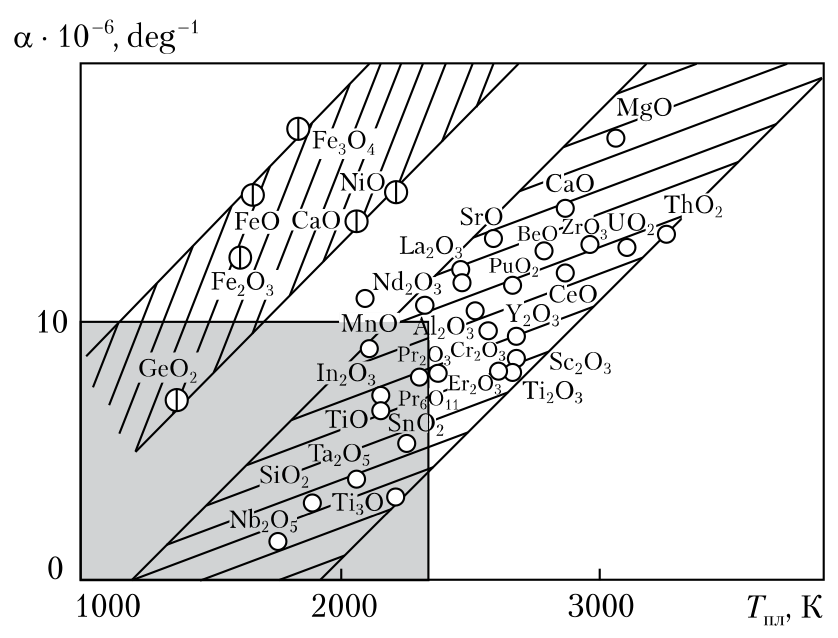
features, the analysis of which can be useful, for example, for explaining the behavior of ceramics when heated or for choosing oxides to modify the surface of grains with oxides. Given the experimental data obtained by the authors [12] on the introduction of oxides into the con-tact zone of the grinding wheel and the machined product, as well as the correlation dependences obtained above, it has been determined that for the modification of the grains of grinding powders, the use of oxides with a CTE higher than 10 10–6, deg–1 is impractical. Other limitations, ac-cording to the authors, are limitations on the melting temperature of such oxides (at most, 2300 K, see Fig. 5), on the specific molar heat capacity (at most, 80 J/(mol K) and on the density of oxides (at, most 5 103 kg/m3 [13].
Given the above limitations, the oxides that can be used in the modification of the surface of diamond grains with heat-resistant oxides should include a significant part of the oxides of the MeO2 group (TiO2, SiO2 , GeO2 , SnO2), while the oxides of Me2O3 (N2O3 , B2O3, Al2O3) and MeO (TiO, BaO, BeO, CaO) groups may be lesser em-ployed [13].
Considering the relevance of the problem outlined above, it has been considered appropriate to conduct research on the creation of a technology
for modifying the surface of diamond grains with the above-mentioned oxides or their mixes, the use of which would raise the efficiency of the grinding tool, therefore the purpose of this research is to develop the process of forming heat-stable wear-resistant coatings on the surface of grains of abrasive grinding powders with the use of mixes of soluble and insoluble oxygen-, silicate- and car-bide-containing activated components.
Averaged samples of grinding powders from synthetic diamonds: AC6 125/100 and AC15 250/200 as raw material have been selected for the research. The surface of powder grains has been modified by the isothermal method of liquid-phase application from saturated solutions of heat-resistant oxides (B2O3), chlorides (CaCl2, NaCl, MgCl2, FeCl3), and their mixes (B2 O3 + CaCl2, B 2O3 + + NaCl). The structural and morphological characteristics of the external structure and the quantitative elemental composition of the modified powders have been determined with the use of a ZEISS EVO 50XVP scanning electron microscope (SEM) equipped with an INCA ENERGY 450 energy dispersive X-ray spectrum analyzer.
The modification of the surface of powder materials by the method of liquid-phase application is the process of deposition of a modifier substance that is released (crystallized) from a solution in the form of crystals or films, on the surface of a solid body (in our case, diamond grinding powder). The modifier is fixed on the grains of such a powder due to the process of physical adsorption, the phenomenon of absorption of gases, salts, or other substances from solutions by some solid bodies. The active centers that exist on the surface of the synthetic diamond grains are the primary centers of fixation of the modifier. In our physical adsorption (modification) process, the adsorbent is synthetic diamond grinding powder, the adsorbate is the deposited layer of the modi-fi er substance (B2O3/NaCl/CaCl2/…), and the substance to be adsorbed (adsorptive) is the saturated solution of the modifier substance. In the liquid-phase method of the formation of deposit-ed layer from a solution of heat-resistant compounds (for example, B2O3, CaCl2, MgCl2, FeCl3, etc.), when the substance is deposited on the sur-face of the grains of the powder material, the sub-stance gets crystallized.
This occurs in the case of a saturated solution and is positive, as it helps to achieve a sufficient thickness of the deposited layer. As our research has shown, even with a short duration of the modifi cation process, the size of the formed crystals is signifi cantly smaller (by one or two orders of magnitude) as compared with the size of the diamond grains.
We have developed the basic technology for li-quid-phase formation on grains of diamond grin-ding powders of combined heat-stable wear-resist-ant coatings, i.e. the modifi cation of the surface of diamond grains. The composition of the coating includes oxygen- (B2O3, TiO2, SiO2, Al2O3, TiO, CaO, ZnO, CeO2, SnO2), silicate- (Na2O(SiO2)n, (K2O(SiO2)n), and carbide-containing compounds (SiC, TiC, B4C) in various combinations. Initial-ly, the insoluble components are activated by the mechanochemical method. First, a saturated aque-ous solution of boric anhydride (B2O3) is prepa-red. 0.3—0.5 g of activated insoluble oxide powder (TiO2 SiO2, Al2O 3, TiO, CaO, ZnO, CeO2, SnO2) and/or insoluble carbide (SiC, TiC, B4C) is add-ed to 10—15 ml of the solution. The diamond powder (the weight of the powder sample for the experiment is 25—30 ct) to be coated is mixed with 10—15 ml of a saturated solution of the mod-ifi er substance (boric anhydride and insoluble ox-ide and/or carbide) with the use of a magnetic stirrer for 10 min under normal conditions. The excess solution is poured off , with the remaining mix fi ltered. The precipitate on the fi lter is added to the bulk of the modifi ed powder. Finally, we dry the obtained wet powder, while stirring, at a temperature of 120 C until get a dry, homoge-neous state.
Having applied the coating, we determine the rel-ative amount of the modifi er substance by the gravi-metric method, as well as the change in the heat re-sistance of the coated (modifi ed) powders. Both the original and the modifi ed samples are treated with heat in the air in a tube furnace at a temperature
of 900 C for 1 h. The samples are weighed before and after heating, with the heat resistance coeffi-cient K ts determined on the basis of the weighing results. So, Kts for the original AC6 125/100 abra-sive powder is 0.55, while, for the same abrasive powder modifi ed with a mix of soluble and insol-uble oxides B2O3 + Al2O3 (or SiO2, TiO2, SnO2, CeO2; CaO, ZnO), it ranges 0.95—0.97.The average coverage of diamond grains, for example, by boron oxide (B2O3) is 5.64% (the B2O3 density is 2.55 g/cm3, the diamond density is 3.56 g/cm3).
Table 1. Quantitative Element Composition (% wt.) of the Studied Diamond Grains
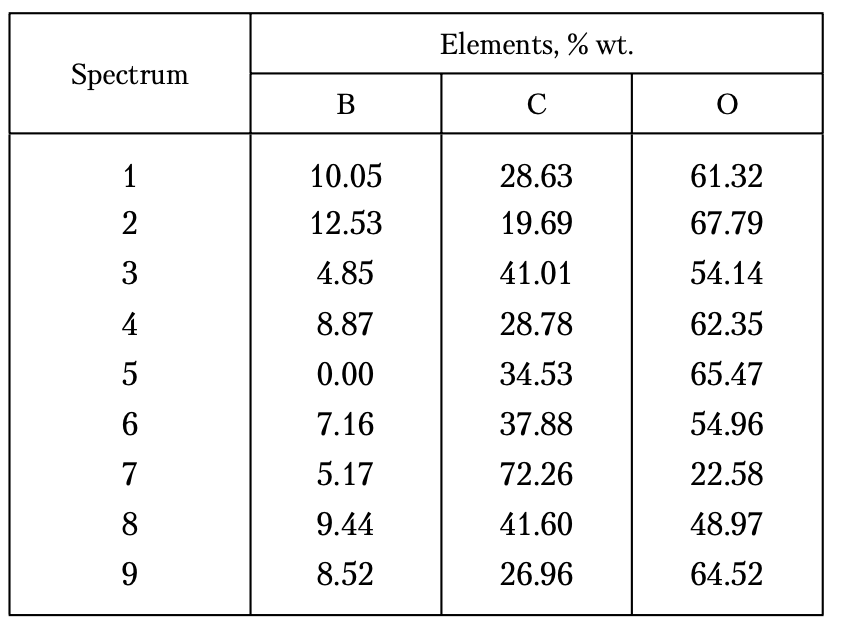
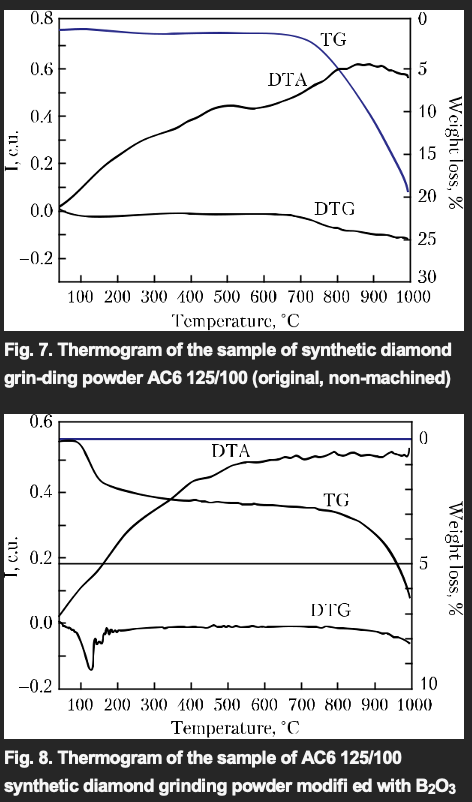
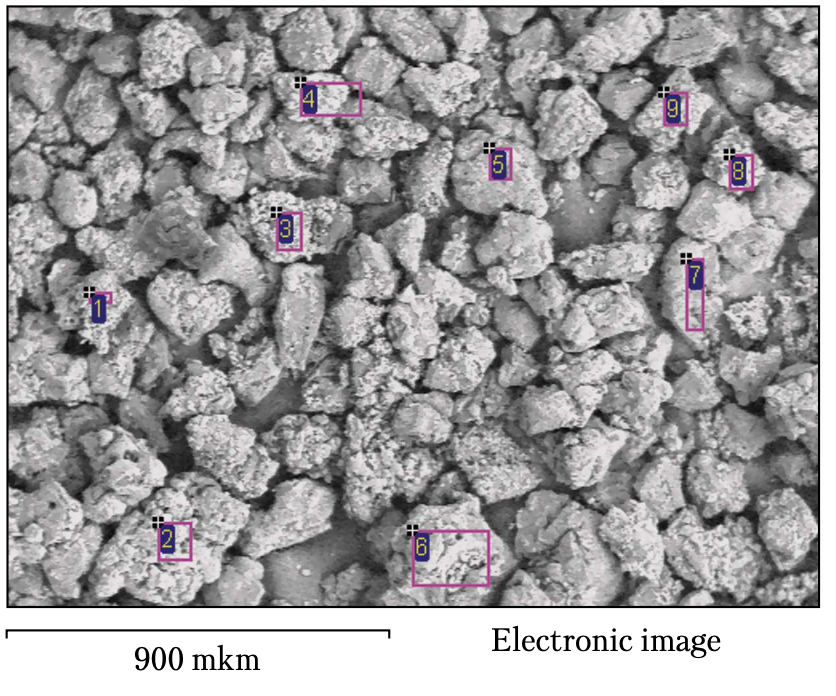
Figure 6 shows a sample of AS6 125/100 dia-mond grinding powder that is three times modified with B2O3 oxide and the regions in which the elemental composition of the grain surfaces of the sample is determined by the method of local X-ray spectral (LXRS) analysis.
The results of the quantitative LXRS analysis of the sample (three times modified with B2O3) (Table 1) have shown that the content of carbon (diamond) ranges from 72.26 to 19.69%, that of boron varies from 0.0 to 12.53%, and that of oxygen ranges from 22.58 to 67.79%.
The indicated diamond grinding powders have been examined by the method of differential thermal analysis on the Q-1500 D derivatograph. Figures 7 and 8 present the results of thermo-gravimetric, differential thermogravimetric, and differential thermal analysis of AS6 125/100 syn-thetic diamond grinding powder samples: the original sample and the sample with the diamond grain surface modified with B2O3.
The weight of the samples is 150 mg, the heating rate is 10 /min. The weight loss after complete cooling of the furnace: 26.3%, for the original sample, and 7.5%, for the modified sample, i.e. the weight loss of the modified sample is 3.5 times less as compared with the original one. Thus, based on the analysis of the research results, it is possible to state that the modification of the diamond surface with oxides increases the heat re-sistance of synthetic diamond grinding powders.
Table 2. Operational Indicators of Diamond Grinding Wheels on Polymer
Bond B2-08 with a Relative Concentration of Grains of 100%, with Various Options for Surface Modification of AS6 125/100 Diamond Grains for Grinding Hard Alloy T15K6 with a Productivity of 400 mm3/min
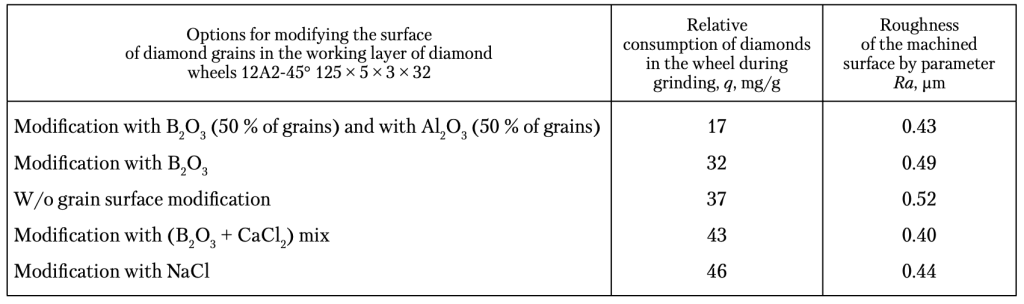
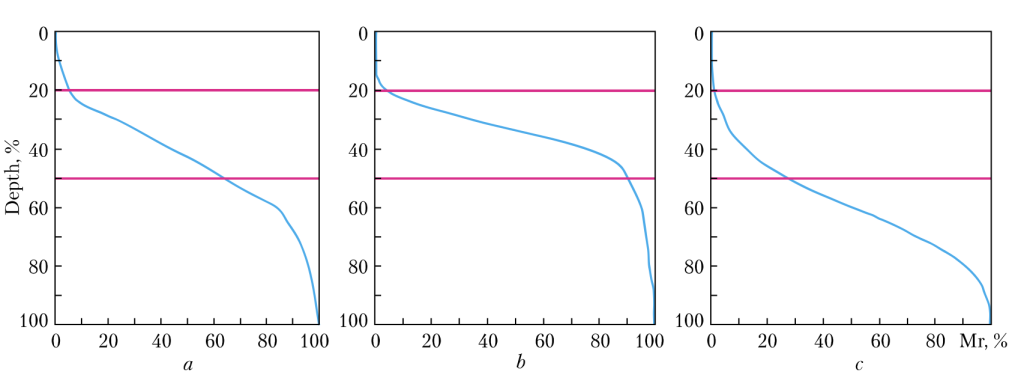
Fig. 9. Dependence of the relative reference length of the profi le of the surface machined by a wheel without diamond grain surface
modifi cation (a), by a wheel with modifi cation of the grain surface by a combination of B2O3/Al2O3 (b), and by a wheel with
modifi cation of the grain surface with NaCl (c), during grinding of a hard alloy with a productivity of 400 mm3/min
Further, we have investigated the operational characteristics of diamond wheels with grinding powders of AS6 synthetic diamond with the above mentioned surface modifications. For machining, we shave elected hard-workable tungsten-titanium-cobalt T15K6 hard alloy with sample dimensions of 63 × 15 × 7 mm. The grinding conditions are as follows: the wheel rotation speed is 18 m/s, the transverse feed is 0.05 mm/revolution, the longitudinal feed is 0.57 m/min (at a productivity of 200 mm3/min) and 1.14 m/min (at a productivity of 400 mm3/min). The wear resistance of the grinding tool is evaluated according to the indicator of relative consumption of diamonds (q) and the roughness of the machined surface according to the indicator (Ra). The test results are shown in Table 2. The Table data show that the modification al-lows reducing the consumption of diamonds in the grinding wheels, with the modification with B2O3 and Al2O3 oxides being the best option. At the same time, the modification of the diamond grain surface with chlorides worsens the wear resistance of the diamond tool. The difference in the diamond wheel wear for the option without modification and that with surface modification with B2O3/Al2O 3 is 2.18. That is, the modification of the surface of diamond grains with a combination of B2O3/Al2O3 guarantees a double increase in the wear resistance of diamond grinding wheels. In general, even despite a certain increase in the cost of diamond grains by approximately 9—11%, due to the introduction of an additional operation — the modification of their surface — the modification of diamond grains by the B2O3/Al2O3 combination leads to, at least, a double increase in the wear resistance of grinding wheels. Now, let us consider the roughness of the ma-chined surface (see Table). It can be seen that in all cases of the modification, the roughness (Ra parameter) decreases. The modifiers that have the effect of reducing the Ra parameter can be arranged in the Ra ascending order as: B2O3/CaCl2 – B2O3/Al2O3 – NaCl – B2O3. In addition, we have found that, if necessary, changing the surface modifier of diamond grains, it is possible to influence the holding capacity of the rough surface obtained during the grinding (Fig. 9). Figure 9 shows that the modification of the diamond grain surface with combination of B2O3/ Al2O3 allows increasing the filling of the rough surface with material (the larger the surface area ISSN 2409-9066. Sci. innov. 2024. 20 (1) 11 Lavrinenko, V. I., Bochechka, O. O., Poltoratskyi, V. G., Smokvyna, V. V., and Solod, V. Yu. under the curve of the relative reference length of the profile, the higher the filling) and enhancing its holding capacity. The modification of the diamond grain surface with NaCl leads to a significant decrease in the density of the rough layer and the holding capacity of the surface. The latter can be used in the case when it is necessary to quickly refine the surface of a part.
CONCLUSIONS
1. The analysis of literature data has proven that various oxides have been used to modify the surface of diamond grains: WO2, NiO and CuO, Al2O3, SiO2, and B2O3, which mainly contribute to the protection of diamond grains from oxidation, both in the manufacture of grinding wheels and in the process of diamond grinding.
2. The basic technology for the formation of combined liquid-phase thermostable wear-resist-ant coatings on the grains of diamond grinding powders, i.e., the modifi cation of the diamond grain surface has been developed. The composition of the coating includes oxygen- (B2O3, TiO2, SiO2, Al2O3, TiO, CaO, ZnO, CeO2, and SnO2), sili-cate- (Na2O(SiO2)n, K2O(SiO2)n), and carbide-containing compounds (SiC, TiC, B4C) in various combinations. The insoluble components are pre-activated by the mechanochemical method.
3. Heat treatment of both original and modifi ed samples is carried out in the air environment, in a tube furnace at a temperature of 900 C for 1 h. The samples are weighed before and after heating, with the heat resistance coefficient Kts determined based on the weighing results. So, for the original AC6 125/100 abrasive powder, the Kts value is equal to 0.55, while for the same abrasive powder modifi ed with a mix of soluble and insoluble oxi- des B 2O3 + Al2O3 (or: SiO2, TiO2, SnO2, CeO2; CaO, and ZnO), Kts ranges within 0.95—0.97.
4. It has been established that the modifi cation allows reducing the consumption of diamonds in the grinding wheels, with the modifi cation by B2O3 and Al2O3 oxides being the best option. At the same time, the modifi cation of the diamond grain surface with chlorides worsens the wear re-sistance of the diamond tool. The ratio of the dia-mond wheel wear without modifi cation to that with surface modifi cation by B2O3/Al2O3 is 2.18. That is, the modifi cation of the surface of dia-mond grains with a combination of B2O3 /Al2O3 guarantees a double increase in the wear resist-ance of diamond grinding wheels.
5. Although the modifi cation of the surface of diamond grains leads to a slight increase (by 9— 11%) in the cost of diamond grains due to the in-troduction of an additional operation, but this increase is compensated by a signifi cant (at least, doubles) increase in the wear resistance of expen-sive diamond wheels.
6. It has been established that in all cases of the modifi cation, the roughness in terms of the Ra parameter decreases. The modifi ers that have the eff ect of reducing the Ra parameter can be arran-ged in the Ra ascending order as follows: B2O3/ CaCl2 — B2O3/Al2O3 — NaCl — B2O3.
7. The modifi cation of the surface of diamond grains with combination of B2O3/Al2O3 allows in-creasing the fi lling of the rough surface with ma-terial (the larger the surface area under the curve of the relative reference length of the profi le, the higher the fi lling) and enhancing its holding ca-pacity. The modifi cation of the diamond grain surface with NaCl leads to a signifi cant decrease in the density of the rough layer and the holding capacity of the surface. The latter can be used in the case when it is necessary to quickly refi ne the surface of a part. Research funding. The research has been fund-ed from the fundamental research project of the National Academy of Sciences of Ukraine for studying the regularities of the formation of com-bined multi-component thermostable wear-re-sistant coatings on the surface of abrasive grind-ing powder grains (state registration number 0121U100622, 2021—2023).
REFERENCES
1. Lavrinenko, V. I., Ilnitskaya, G. D., Sheiko, M. N., Dobroskok, V. L., Ostroverkh, Ye. V., Solod, V. Yu. (2021). Improving the performance characteristics of synthetic diamond for high-precision diamond dressing tool. Science and innovation, 17(6), 72—82. https://doi.org/10.15407/scine17.06.072.
2. Baoyan Liang, Zhen Dai, Qi Zhang, Wangxi Zhang, Ruij ie Zhang, Ying Liu, Jizhou Zhang, Li Ya (2021). Coating of dia-mond by thermal explosion reaction. Diamond and Related Materialі, 119 (November 2021), 108572. https://doi.org/ 10.1016/j.diamond.2021.108572.
3. Ukhina, A. V., Dudina, D. V., Bokhonov, B. B., Savintseva, D. V., Samoshkin, D. A., Stankus, S. V. (2022). Morphological features and phase composition of W-containing coatings formed on diamond via its interaction with WO3. Diamond and Related Materials, 123, 108876. https://doi.org/10.1016/j.diamond.2022.1088762.
4. Ao Deng, Jing Lu, Dongxu Li, Yanhui Wang. (2021). Exploring the activation energy of diamond reacting with metals and metal oxides by fi rst-principle calculation. Diamond and Related Materials, 118, 108522. https://doi.org/10.1016/j. diamond.2021.108522.
5. Dominguez, D., Tiznado, H., Borbon-Nuñez, H. A., Muñoz-Muñoz, F., Romo-Herrera, J. M., Soto, G. (2016). Enhancing the oxidation resistance of diamond powder by the application of Al2O3 conformal coat by atomic layer deposition. Dia-mond and Related Materials, 69, 108—113. https://doi.org/10.1016/j.diamond.2016.08.005.
6. Kongping Wu, Yong Zhang, Jianli Ma, Zhifen Fu, Changzhao Chen. (2020). Two-dimensional hole gas formed at dia-mond surface by Al2O3/diamond interface engineering. Diamond and Related Materials, 105, 107807. https://doi. org/10.1016/j.diamond.2020.107807.
7. Naichao Chen, Fasong Ju, Fan Zhou, Shuai Chen, Kun Wei, Ping He. (2021). Growth and characterization of chemical vapor deposition diamond coating incorporated (amorphous carbon with high Raman bands induced by CuO particles. Diamond and Related Materials, 116, 108387. https://doi.org/10.1016/j.diamond.2021.108387.
8. Jing Lu, Yongchao Xu, Yunhe Zhang, Xipeng Xu. (2017). The eff ects of SiO2 coating on diamond abrasives in sol-gel tool for SiC substrate polishing. Diamond and Related Materials, 76, 123—131. https://doi.org/10.1016/j.diamond.2017.05. 003.
9. Congxu Zhu, Can Cui, Xiwang Wu, Bowen Zhang, Dong Yang, Hongxiao Zhao, Zhi Zheng. (2020). Study on surface modifi cation of diamond particles and thermal conductivity properties of their reinforced metal-based (Cu or Mg) com- posides. Diamond and Related Materials, 108 (October 2020), 107998. https://doi.org/10.1016/j.diamond.2020.107998.
10. Herrmann, M., Matthey, B., Gestrich, T. (2019). Boron-doped diamond with improved oxidation resistance. Diamond and Related Materials, 92 (February 2019), 47—52. https://doi.org/10.1016/j.diamond.2018.12.001.
11. Youhong Sun, Chi Zhang, Jinhao Wu, Qingnan Meng, Baochang Liu, Ke Gao, Linkai He (2019). Enhancement of oxida-tion resistance via titanium boron carbide coatings on diamond particles. Diamond and Related Materials, 92 (February 2019), 74—80. https://doi.org/10.1016/j.diamond.2018.12.019.
12. Lavrinenko, V. I., Solod, V. Yu. (2016). Oxidation or oxide materials in the machining zone in superabrasive grinding – a factor of infl uence on the grinding performance. J. Superhard Mater., 38(6), 417—422. https://doi.org/10.3103/S106345761606006X.
13. Lavrinenko, V. I., Solod, V. Yu., Kashynskyi, I. S., Dobroskok V. L. (2020). Determination of oxides intended for the surface modifi cation of diamond grains by the functional characteristics. Journal of Superhard Materials, 42(6), 417—422. https:// doi.org/10.3103/s1063457620060064.
